The technology
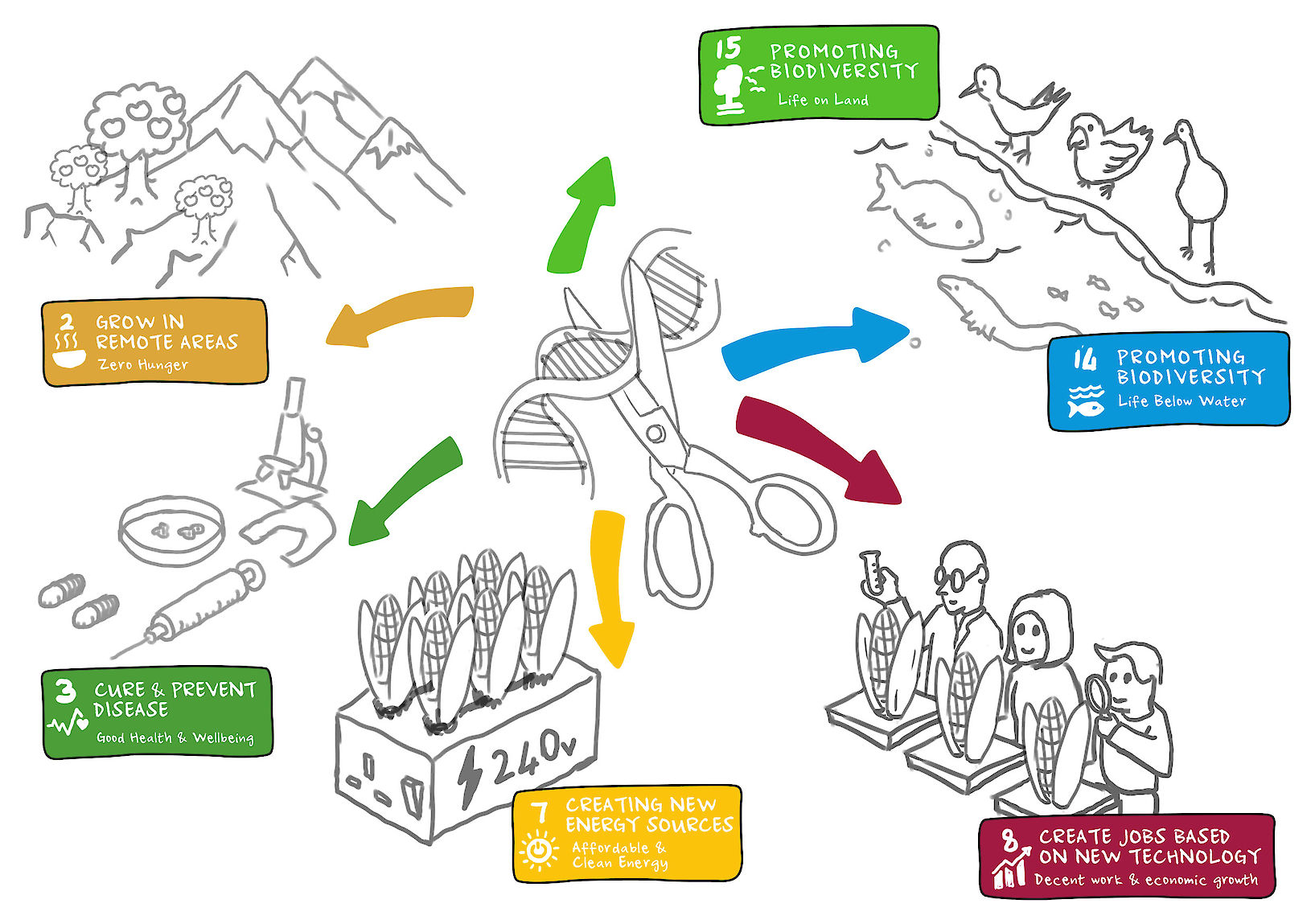
The leading technology, CRISPR (clustered regular interspaced short palindromic repeats) is a highly cost effective gene editing technology (estimated, by Professor James Haber, Brandeis University, to be 150 times cheaper than other leading technologies). It is also easy to use requiring less training and education than other technologies. The use of CRISPR makes it possible to locate and edit specific spots on any DNA by disabling or knocking out genes, patching in a new gene, or replacing an existing gene.
The potential
In the short term, gene editing technology could be used to edit T-cells (white blood cells critical to the immune system) to better recognise cancer, or to edit immune cells to mimic a rare, naturally occurring immunity to HIV. This could turn these often terminal conditions to chronic or curable ones.
In the longer term, this work could identify and cure debilitating genetic disorders by editing these defects out of the patient’s DNA entirely. This would mean they would never become ill from a known genetic cause.
The barriers
By directly editing DNA this technology has the ability to pass traits on to future generations, and the potential to cause irreversible harm to human health and the environment, whether as the result of intentional misuse or a lack of understanding. For this reason, risks need to be understood and mitigated in order to enable safe adoption and positive benefits.